I will separately post on the next gen, once daily combo tab for Hep C Merck is advancing through Phase III, now. FDA has received the packet, and will likely make a decision on it early in 2016. However, Kenilworth already faces some daunting competitors in this space. Just the same, this is moderately good news:
. . . .Separately, Merck said, the U.S. Food and Drug Administration has accepted for review the company’s new drug application for grazoprevir/elbasvir, a once-daily, single-tablet therapy for hepatitis C. The agency granted priority review for the treatment with a decision expected by Jan. 28 [2016]. . . .
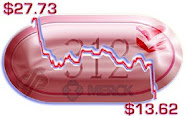
And, just as expected Remicade tok a hit from biosim introductions this quarter. That will accelerate in future quarters. Onward. . . .















No comments:
Post a Comment