To be fair, Rob Davis reaching $20 million plus in annual compensation is primarily a function of the rising price of Merck's stock on the NYSE (and that has been
very good for all shareholders, when added to the very hefty dividends, paid every quarter, year after year -- since at least the 1950s). You see, at the time these last grants of options and restricted stock were made, Merck was trading between $95 and $103 per share.
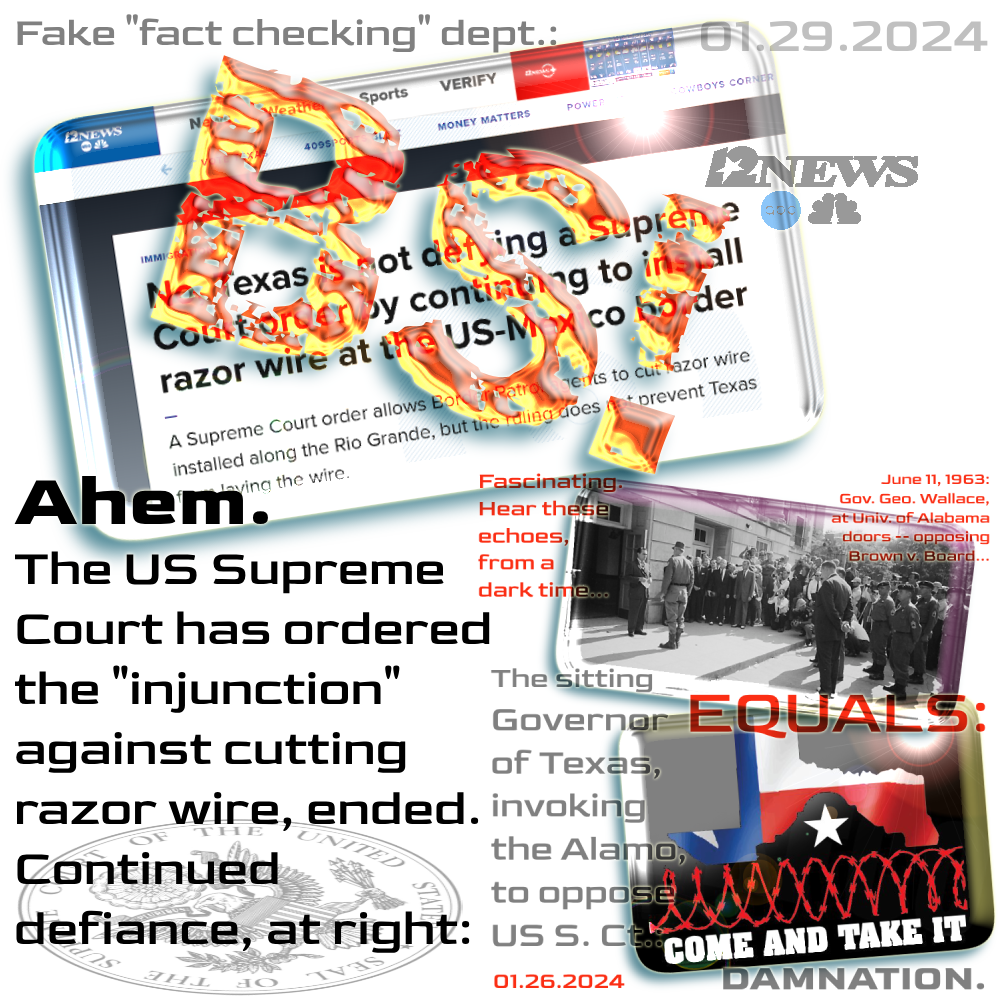
Recently Merck's NYSE price was trading between $128 and $134 a share -- up smartly from these latest grant dates. However, Mr. Davis's cash bonus was decreased, as it has probably maxed out -- in its "
added carrot" appeal to him, given that he is up over 30%, on his stock compensation. But the board did bump up his salary to run a little ahead of the core inflation rate for the year. [Not that he would be, in any manner, worried about the rising prices of avocados -- in terms of daily brown bag lunch affordability -- mind you.]
All in, while I had complained for years that the con-man "Fast" Fred Hassan was vastly overpaid -- for his uniformly terrible decision-making, at legacy Schering-Plough. . . I generally felt that Kenneth C. Frazier was fairly compensated for the unique skill set he offered Merck.
So too now, Mr. Davis -- he's matured as a leader since his early days at Lilly. That said, he lacks some of the charisma of a Ken Frazier or Roy Vagelos (perhaps the gold standard name in this arena). Here are the details, from
Fierce's nice collection of proxy disclosures -- as Saturday filler fodder:
. . .Merck CEO Robert Davis has cracked the $20-million mark in annual pay for the first time, putting him in an exclusive club of biopharma heavy hitters.
Davis, 57, who took over as CEO in 2021 and as chairman the following year, received a 9% bump in pay in 2023 to $20.3 million, according to the company’s proxy filing.
Davis joins Johnson & Johnson’s Joaquin Duato ($28.4 million), Eli Lilly’s David Ricks ($26.6 million), AbbVie’s retiring Richard Gonzalez ($25.7 million), Pfizer’s Albert Bourla ($21.6 million), AstraZeneca's Pascal Soriot ($21.3 million) and Vertex’s Reshma Kewalramani ($20.6 million) in the $20-million-plus club for 2023. . . .
Davis's compensation increase in 2023 came despite a drop in his bonus from $4.1 million to $3.6 million. His equity awards however increased from $11.4 million to $14 million, while his salary was up from $1.54 million to $1.60 million. . . .

Indeed, though -- it is astonishing that the CEOs of these vastly profitable behemoth companies are just now catching up to what the CEO Jason Les pulled down, last year (last available disclosed total: 2022) at tiny Riot Platforms (revenue of under $100 million; never GAAP profitable), for losing over $2 billion life to date -- and (did I say this part already?!)
never making a GAAP profit from operations in eight long years.
Riot is a Bitcoin miner -- like all the others, set to get crushed by the "
halvening" -- coming end of next week. [Accordingly, it has seen its stock fall from the mid-$20s, to a little over $9 on the NASDAQ, as of Friday.]
That THAT GUY makes over $20 million a year is absolutely. . .
I N S A N E.
नमस्ते